
Huntington’s Disease Clinical Trial Pipeline Analysis: 20+ Key Companies Shaping the Future of Dopamine Receptor Antagonists Therapeutics | DelveInsight
The Huntington's disease treatment market is poised for significant growth, driven by rising prevalence rates and increased awareness globally. Advancements in gene-targeting and disease-modifying therapies, along with a robust clinical pipeline, are addressing critical unmet needs. Active government support and educational initiatives are further enhancing diagnosis and treatment uptake. Together, these factors create a strong foundation for market expansion in the coming years.
/EIN News/ -- New York, USA, June 03, 2025 (GLOBE NEWSWIRE) -- Huntington’s Disease Clinical Trial Pipeline Analysis: 20+ Key Companies Shaping the Future of Dopamine Receptor Antagonists Therapeutics | DelveInsight
The Huntington's disease treatment market is poised for significant growth, driven by rising prevalence rates and increased awareness globally. Advancements in gene-targeting and disease-modifying therapies, along with a robust clinical pipeline, are addressing critical unmet needs. Active government support and educational initiatives are further enhancing diagnosis and treatment uptake. Together, these factors create a strong foundation for market expansion in the coming years.
DelveInsight’s 'Huntington’s Disease Pipeline Insight 2025' report provides comprehensive global coverage of pipeline Huntington’s disease therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the Huntington’s disease pipeline domain.
Key Takeaways from the Huntington’s Disease Pipeline Report
- DelveInsight’s Huntington’s disease pipeline report depicts a robust space with 20+ active players working to develop 20+ pipeline Huntington’s disease drugs.
- Key Huntington’s disease companies such as Hoffmann-La Roche, Medibiofarma, PTC Therapeutics, Annexon, Alnylam Pharmaceuticals, Neuvivo, Skyhawk Therapeutics, BPG Bio, and others are evaluating new Huntington’s disease drugs to improve the treatment landscape.
- Promising pipeline Huntington’s disease therapies, such as RG6042, MBF 015, PTC518, ANX-005, ALN-HTT02, NP001, SKY 0515, Research Programme: HDD, and others are in different phases of Huntington’s disease clinical trials.
- In April 2025, the FDA granted a breakthrough therapy designation to AMT-130 (uniQuire) for the treatment of Huntington's disease. Previously, AMT-130 was also granted a regenerative medicine advanced therapy designation and orphan drug designation for this indication.
- In April 2025, Prilenia Therapeutics announced that it had entered into a collaboration and license agreement with Ferrer for the development and commercialization of pridopidine in Europe and other select markets.
- In March 2025, Skyhawk Therapeutics presented their novel SKY-0515 small molecule RNA splicing modulator targeting Huntington's Disease to members of the Huntington's Disease Youth Organization (HDYO), at the biannual HYDO International Congress in Prague, Czech Republic.
- In December 2024, Novartis announced that it had entered into a global licensing and collaboration agreement with PTC Therapeutics for PTC518, an HTT mRNA splice modulator for the treatment of Huntington's disease. Under the terms of the agreement, Novartis will pay USD 1 billion upfront and up to USD 1.9 billion in development, regulatory, and sales milestones. Novartis will also share profits in the US and pay tiered royalties on sales outside the US.
- In September 2024, PTC Therapeutics announced that the FDA had granted Fast Track designation to the PTC518 program for the treatment of Huntington's disease.
- In September 2024, the European Medicines Agency (EMA) accepted the marketing authorization application (MAA) for Prilenia Therapeutics’ pridopidine for the treatment of adults with Huntington's disease.
Request a sample and discover the recent advances in Huntington’s disease drugs @ Huntington’s Disease Pipeline Report
The Huntington’s disease pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage Huntington’s disease drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the Huntington’s disease clinical trial landscape.
Huntington’s Disease Overview
Huntington’s disease is a hereditary, progressive neurodegenerative condition marked by the gradual onset of involuntary muscle movements—affecting the hands, feet, face, and trunk—and a steady decline in cognitive abilities and memory. It impacts the central region of the brain, leading to difficulties in movement, emotional regulation, and cognitive functions. Symptoms often appear between ages 30 and 50 but can emerge as early as age 2 or as late as 80. A hallmark feature of the disease is the presence of rapid, uncontrolled movements such as muscle jerks or tics. As the condition advances, patients may experience loss of coordination, speech difficulties, memory deterioration, and worsening choreiform movements, along with personality and behavioral changes.
Huntington’s disease follows an autosomal dominant inheritance pattern, meaning only one copy of the faulty gene is needed to cause the disease. It is triggered by a mutation in a single gene on chromosome 4, which encodes the huntingtin protein. While the exact role of huntingtin remains unclear, its mutated form disrupts normal brain function, leading to involuntary movements, severe cognitive decline, and emotional disturbances such as depression and irritability. The mutation involves an expansion of a CAG repeat segment within the gene. Typically, this segment repeats 17 to 20 times in a healthy gene, but in Huntington’s disease, the number of repeats rises to 40 or more.
Diagnosing Huntington’s disease involves a comprehensive clinical assessment, patient history, and several specialized tests. These may include blood work, genetic testing to detect mutations in the HTT gene, and brain imaging techniques such as CT scans, MRI, and EEG. CT and MRI provide detailed cross-sectional images of the brain, while EEG measures the brain’s electrical activity to support diagnosis.
Currently, there is no cure for Huntington’s disease, and treatment is aimed at alleviating symptoms. The only two FDA-approved medications—Austedo and Xenazine—are indicated specifically for managing chorea related to Huntington’s. Additional symptom management may include antidepressants, antipsychotics, and mood stabilizers to address psychiatric symptoms. Supportive therapies and multidisciplinary care are also critical in improving the quality of life for individuals affected by this condition.
Find out more about Huntington’s disease drugs @ Huntington’s Disease Treatment
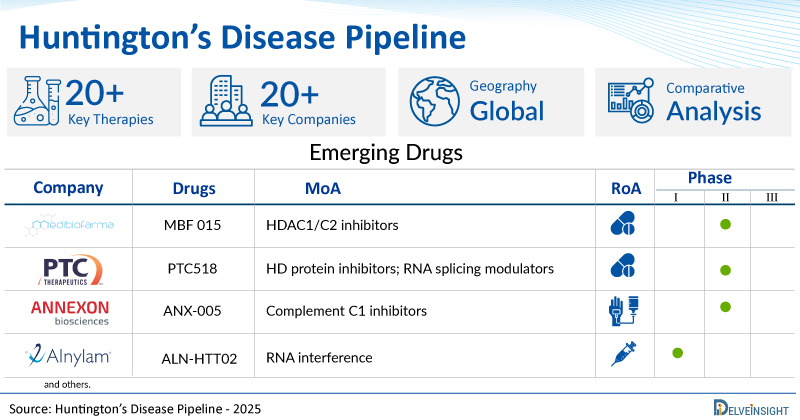
A snapshot of the Pipeline Huntington’s Disease Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| MBF 015 | Medibiofarma | II | HDAC1/C2 inhibitors | Oral |
| PTC518 | PTC Therapeutics | II | HD protein inhibitors; RNA splicing modulators | Oral |
| ANX-005 | Annexon | II | Complement C1 inhibitors | Intravenous |
| ALN-HTT02 | Alnylam Pharmaceuticals | I | RNA interference | Intrathecal |
| NP001 | Neuvivo | I | Macrophage modulators | Unspecified |
| SKY 0515 | Skyhawk Therapeutics | I | RNA splicing modulators | Oral |
Learn more about the emerging Huntington’s disease therapies @ Huntington’s Disease Clinical Trials
Huntington’s Disease Therapeutics Assessment
The Huntington’s disease pipeline report proffers an integral view of the emerging Huntington’s disease therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Huntington’s Disease Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Therapeutics Assessment By Mechanism of Action: HDAC1/C2 inhibitors, RNA interference, HD protein inhibitors, RNA splicing modulators, Macrophage modulators, Complement C1 inhibitors
- Key Huntington’s Disease Companies: Hoffmann-La Roche, Medibiofarma, PTC Therapeutics, Annexon, Alnylam Pharmaceuticals, Neuvivo, Skyhawk Therapeutics, BPG Bio, and others.
- Key Huntington’s Disease Pipeline Therapies: RG6042, MBF 015, PTC518, ANX-005, ALN-HTT02, NP001, SKY 0515, Research Programme: HDD, and others.
Dive deep into rich insights for new Huntington’s disease treatments, visit @ Huntington’s Disease Drugs
Table of Contents
| 1. | Huntington’s Disease Pipeline Report Introduction |
| 2. | Huntington’s Disease Pipeline Report Executive Summary |
| 3. | Huntington’s Disease Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Huntington’s Disease Clinical Trial Therapeutics |
| 6. | Huntington’s Disease Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Huntington’s Disease Pipeline: Late-Stage Products (Phase III) |
| 8. | Huntington’s Disease Pipeline: Mid-Stage Products (Phase II) |
| 9. | Huntington’s Disease Pipeline: Early-Stage Products (Phase I) |
| 10. | Huntington’s Disease Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Huntington’s Disease Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Huntington’s Disease Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the Huntington’s disease pipeline therapeutics, reach out @ Huntington’s Disease Therapeutics
Related Reports
Huntington’s Disease Epidemiology Forecast
Huntington’s Disease Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted Huntington’s disease epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Huntington’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Huntington’s disease companies, including Prilenia Therapeutics, Neurocrine Biosciences, SOM Biotech, Annexon Biosciences, Vaccinex, Sage Therapeutics, UniQure Biopharma, Wave life sciences, Takeda, Medesis Pharma, among others.
Wilson Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Wilson disease companies, including Alexion Pharmaceuticals, Vivet Therapeutics, Orphalan, among others.
Wilson Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Wilson disease companies, including Alexion Pharmaceuticals, Vivet Therapeutics, Orphalan, among others.
Bipolar Disorder Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key bipolar disorder companies, including Vanda Pharmaceuticals, Pear Therapeutics, Sunovion, Lyndra Therapeutics, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

